What is PCOS?
Polycystic ovary syndrome (PCOS) is an endocrine disorder which features amenorrhea, hyperandrogenaemia, and enlarged ovaries with multiple cysts (Sirmans & Pate, 2013). PCOS has shown to be present in over 10% of women of childbearing age and in severe cases causing infertility (March et al, 2010). More than 50% of women with PCOS are obese (Butterworth, Deguara & Borg, 2016). To improve quality of life for these women many lifestyle altercations are required however this only works to an extent (Harris-Glocker et al, 2010). For women with a body mass index (BMI) of over 40kg/m2 or a BMI of over 35kg/m2 with an obesity related condition which would be improved with weight loss (such as diabetes type 2 or high blood pressure), bariatric surgery is a solution (nhs.uk, 2017). Pathophysiology mechanisms of PCOS in relation to amenorrhea (the absence of menstruation) will be considered along with current drug interventions. How bariatric surgery affects the fertility of PCOS patients will also be discussed in further depth.
How PCOS leads to amenorrhea
PCOS patients who are obese exhibit impaired ovulation and decreased pregnancy rates relative to PCOS patients at healthy BMI’s (Tziomalos and Dinas, 2018). The basic lesion of PCOS is an endocrinological disturbance within the ovary, which has been associated with excess androgens and other extra-ovarian hormonal abnormalities such as hyperinsulinemia, hirsutism and increased LH:FSH ratio (Palomba, 2018) as seen in figure 1. However individually these endocrinological associations with PCOS do not explain the pathogenesis, suggesting there are wider causes and contributions to the mechanism pathway (Palomba, 2018).
The pathophysiology of PCOS is not yet fully understood however Rotstein (2013) proposes that hyperandrogenaemia is causing an arrest in antral follicle development, which leads to anovulation and so there is no corpus luteum, resulting in subfertility or amenorrhea (Rotstein, 2013) (figure 1). A more recent study by Butterworth, Deguara & Borg (2016) suggests that insulin resistance and hyperinsulinemia are the key pathophysiological mechanisms (Butterworth, Deguara & Borg, 2016). Although the complex interaction between environment, genetics and lifestyle have heavy impacts on fertility, the exact aetiology is still unknown (Butterworth, Deguara & Borg, 2016). Intrauterine androgen exposure is in question as a contributing factor of anovulation, as Dumesic et al (2018) has shown that pregnant women with PCOS have elevated testosterone, but it is not yet clear if the foetus is exposed to the increased androgen levels (Dumesic et al, 2018). A study investigating the aromatase activity of the placenta would provide insight and potentially confirm if intrauterine androgen exposure directly contribute to anovulation of PCOS patients (Palomba, 2018). If this mechanism is more understood then a suitable intervention may be able to prevent foetal androgen exposure.
To combat subfertility, the first-line drug therapy used to induce ovulation is oral anti-oestrogen clomiphene citrate (CC) or letrozole (Balen et al, 2016). CC works by blocking hypothalamic and pituitary oestrogen receptors, it also induces a discharge of FSH which triggers regular ovulation (Homburg and Filippou, 2016). Insulin resistance is a common feature of PCOS, there has shown to be a correlation between insulin resistance and CC resistance (Kar, 2012). In such cases of insulin resistant PCOS patients, letrozole was a suitable alternative (Kar, 2012). Letrozole is an aromatase inhibitor which acts by reducing oestrogen production by blocking androgens which would balance the androgen levels, preventing the subfertility (Kar, 2012). As figure 1 portrays, metformin is also a common drug used to reduce hyperinsulinemia and ameliorate hyperandrogenism in obese and non-obese patients (Sam and Ehrmann, 2017). For PCOS patients with insulin resistance, studies reveal that metformin has been successful in inducing regular ovulatory cycles and efficient weight loss (Sam and Ehrmann, 2017). Although not all PCOS patients display insulin resistance therefore metformin drug is specific to PCOS patients with insulin resistance.
CC, letrozole and metformin are effective treatments to induce ovulation however drugs are metabolised differently in patients therefore its effectiveness will vary (Roque et al, 2015). Lifestyle modification remains to be the best first step treatment for amenorrhoeic PCOS patients before resorting to pharmacological ovulation induction (Hashim, 2016). For patients who find lifestyle changes to be ineffective and do not benefit from drug therapy may then consider bariatric surgery.
Reproductive considerations for bariatric surgery
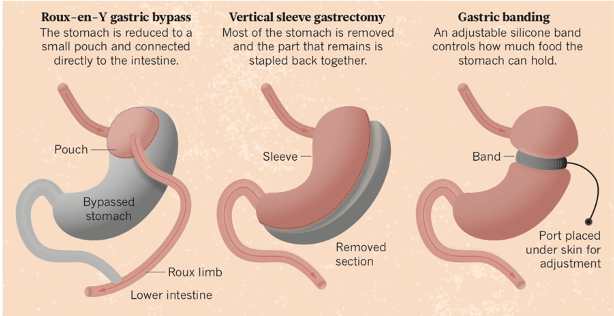
Infertility has shown to be a key reason for PCOS patients seeking bariatric surgery (Christ and Falcone, 2018). PCOS patients were found more proactive in seeking treatment for infertility when compared to amenorrhoeic women without PCOS (Hashim, 2016). Bariatric surgery is a successful management strategy for morbid obesity, however there are limited studies on its effect on patients who exhibit PCOS (Skubleny et al, 2016). PCOS patients are already predisposed to glucose abnormalities and ultimately type 2 diabetes, dyslipidaemia and eventually cardiovascular disease. (Malik and Traub, 2012). Bariatric surgery can be a powerful tool to prevent these fatal consequences and also possibly ameliorate PCOS features (Malik and Traub, 2012). There are three types of bariatric surgery procedures which are most commonly performed (figure 2). These include; Laparospic roux-en-Y Gastric Bypass (LRYGB), Laparospic sleeve gastrectomy (LSG) and Laparospic adjustable gastric banding (LAGB) (Malik and Traub, 2012).
LRYGB has shown to be most effective especially in cases of diabetic patients, where weight loss and glucose control was successful even after a five year follow up (Dicker et al, 2016), however fertility of these patients were not considered in this paper. Jamal et al (2012) highlighted 10 participants who exhibited PCOS and infertility, after a LRYGB procedure the outcomes resulted in menstrual irregularities corrected with a regular cycle of 82% of patients without the need of hormonal treatment or in vitro fertilisation (IVF) (Jamal et al, 2012). Also 60% of their patients had achieved a successful pregnancy within three years postoperative without any pregnancy induced or postpartum complications (Jamal et al, 2012). Malik and Traub (2012) identified in their study a 68% success rate for LRYGB procedures after a 4 year follow up, though this was with limited data and a small sample size (Malik and Traub, 2012). Doblado et al (2010) highlighted a PCOS patient aged 29, LAGB enabled the patient to achieve a successful pregnancy using in vitro fertilisation (IVF). Hughes (2014) described that LAGB has shown to be the least effective as after a three year follow up, patients often lost an average of only 16% of their weight whereas LRYGB enabled a 32% average weight loss (Hughes, 2014). LSG is the newest procedure and so there is limited published results, though results do indicate a higher success rate over LAGB (Malik and Traub, 2012). George and Azeez (2013) found success with LSG where results showed the procedure resolved menstrual dysfunction in 100% of PCOS patients and hirsutism and radiological evidence of PCOS was resolved in 80% of patients (George and Azeez, 2013). Although, George and Azeez (2013) did not have a follow up record therefore it is difficult to determine the long term results from this experiment.
A recent meta-analysis study conducted by Skubleny et al (2016) identified a sample of 2130 obese female patients. 45.6% of these patients exhibited PCOS however after bariatric surgery (mainly LRYGB) there was a significant decrease of PCOS to only 6.8% postoperatively at a one year follow up (Skubleny et al, 2016). Long term data is still limited to confirm if the decreased PCOS symptoms would remain permanently attenuated (Skubleny et al, 2016). The evidence for improvement in fertility after bariatric surgery is still limited but trends do indicate high success rates.
Hopes for the future
The exact relationship between PCOS and amenorrhea remains unknown however strong indications are that hyperandrogenaemia and hyperinsulinemia are key pathophysiology mechanisms (Butterworth, Deguara & Borg, 2016). Lifestyle altercations and drug therapies are effective treatments, however they only work to an extent for some patients (Roque et al, 2015). Several studies indicate that bariatric surgery improves diagnostic features of PCOS in patients (Christ and Falcone, 2018). Though limitations in these studies are substantial, as there are many criteria of infertility which were not mentioned. Infertility is multifactorial where PCOS is a frequent cause (Butterworth, Deguara & Borg, 2016), however almost none of the studies mentioned patient age nor lifestyle choices such as smoking and alcohol intake which all affect fertility. As most studies show amelioration of PCOS postoperatively, the current criteria for bariatric surgery remains to require a BMI of over 35kg/m2 with a condition such as diabetes or hypertension. Infertility should also be considered as a condition for bariatric surgery as there are clear increases in pregnancies postoperatively (Malik and Traud, 2012). A more detailed study on PCOS patients who experience infertility, and an analysis on their age, environmental impacts and genetics would provide further insight into the ambiguity of how bariatric surgery affects fertility of PCOS patients.
References
Balen, A.H., Morley, L.C., Misso, M., Franks, S., Legro, R.S., Wijeyaratne, C.N., Stener-Victorin, E., Fauser, B.C., Norman, R.J. and Teede, H. (2016). The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Human reproduction update, 22(6), pp.687-708.
Butterworth, J., Deguara, J. and Borg, C. (2016). Bariatric surgery, polycystic ovary syndrome, and infertility. Journal of obesity, 2016.
Christ, J. and Falcone, T. (2018). Bariatric Surgery Improves Hyperandrogenism, Menstrual Irregularities, and Metabolic Dysfunction Among Women with Polycystic Ovary Syndrome (PCOS). Obesity surgery, pp.1-7.
Dicker, D., Yahalom, R., Comaneshter, D.S. and Vinker, S. (2016). Long-term outcomes of three types of bariatric surgery on obesity and type 2 diabetes control and remission. Obesity surgery, 26(8), pp.1814-1820.
Doblado, M.A., Lewkowksi, B.M., Odem, R.R. and Jungheim, E.S. (2010). In vitro fertilization after bariatric surgery. Fertility and sterility, 94(7), pp.2812-2814.
Dumesic, D.A., Oberfield, S.E., Stener-Victorin, E., Marshall, J.C., Laven, J.S. and Legro, R.S. (2015). Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocrine reviews, 36(5), pp.487-525.
George, K. and Azeez, H. (2013) August. Resolution of Gynaecological Issues After Bariatric Surgery-A Retrospective Analysis. In Obesity Surgery Vol. 23, No. 8, pp. 1043-1043
Harris-Glocker, M., Davidson, K., Kochman, L., Guzick, D. and Hoeger, K., 2010. Improvement in quality-of-life questionnaire measures in obese adolescent females with polycystic ovary syndrome treated with lifestyle changes and oral contraceptives, with or without metformin. Fertility and sterility, 93(3), pp.1016-1019.
Hashim, H.A., 2016. Twenty years of ovulation induction with metformin for PCOS; what is the best available evidence?. Reproductive biomedicine online, 32(1), pp.44-53.
Homburg, R. and Filippou, P. (2016). Treatment of WHO 2: Clomiphene Citrate. Ovulation Induction: Evidence Based Guidelines for Daily Practice, pg15.
Hughes, V. (2014). A gut-wrenching question. Nature, 511(7509), p.282.
Skubleny, D., Switzer, N.J., Gill, R.S., Dykstra, M., Shi, X., Sagle, M.A., de Gara, C., Birch, D.W. and Karmali, S. (2016). The impact of bariatric surgery on polycystic ovary syndrome: a systematic review and meta-analysis. Obesity surgery, 26(1), pp.169-176.
Jamal, M., Gunay, Y., Capper, A., Eid, A., Heitshusen, D. and Samuel, I. (2012). Roux-en-Y gastric bypass ameliorates polycystic ovary syndrome and dramatically improves conception rates: a 9-year analysis. Surgery for Obesity and Related Diseases, 8(4), pp.440-444.
Kar, S. (2012). Clomiphene citrate or letrozole as first-line ovulation induction drug in infertile PCOS women: A prospective randomized trial. J Hum Reprod Sci. ;5(3):262-5.
Malik, S.M. and Traub, M.L. (2012). Defining the role of bariatric surgery in polycystic ovarian syndrome patients. World journal of diabetes, 3(4), p.71.
March, W.A., Moore, V.M., Willson, K.J., Phillips, D.I., Norman, R.J. and Davies, M.J., (2009). The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Human reproduction, 25(2), pp.544-551.
Nhs.uk. (2017). Weight loss surgery. [online] Available at: https://www.nhs.uk/conditions/weight-loss-surgery/ [Accessed 1 Nov. 2018].
Palomba, S. ed. (2018). Infertility in Women with Polycystic Ovary Syndrome: Pathogenesis and Management. Springer.
Roque, M., Tostes, A.C., Valle, M., Sampaio, M. and Geber, S., 2015. Letrozole versus clomiphene citrate in polycystic ovary syndrome: systematic review and meta-analysis. Gynecological Endocrinology, 31(12), pp.917-921.
Rotstein, A. (2013). Polycystic ovarian syndrome (PCOS) | McMaster Pathophysiology Review. [online] Pathophys.org. Available at: http://www.pathophys.org/pcos/ [Accessed 26 Oct. 2018].
Sam, S. and Ehrmann, D.A., 2017. Metformin therapy for the reproductive and metabolic consequences of polycystic ovary syndrome. Diabetologia, 60(9), pp.1656-1661.
Sirmans, S., and Pate, K. (2013). Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clinical epidemiology, 6, 1-13. doi:10.2147/CLEP.S37559
Tziomalos, K. and Dinas, K., (2018). Obesity and Outcome of Assisted Reproduction in Patients With Polycystic Ovary Syndrome. Frontiers in endocrinology, 9, p.149.